Source | Cybertron Author | Xiao Wan
The varieties of 42 enterprises passed the consistency evaluation, among which Huahai Pharmaceutical, Unacon and Yangzijiang Pharmaceutical were the top three, followed by Hengrui Pharma, Jingxin Pharmaceutical, Haosen Pharmaceutical and Zhengda Tianqing.
42 pharmaceutical companies have reviewed varieties.
Now in mid-November, the overall progress of consistency evaluation has become the most concerned topic for all pharmaceutical companies. A few days ago, according to the incomplete statistics of CyberBlue, a total of 107 product specifications passed the consistency evaluation, among which 42 enterprises passed the consistency evaluation (the number of branches passed was included in the head office).
In statistics, one product specification of 18 pharmaceutical companies passed the consistency evaluation; Two of the 10 pharmaceutical companies have passed the consistency evaluation; Three varieties of seven pharmaceutical companies have been evaluated; Four varieties of three pharmaceutical companies have been reviewed; Four pharmaceutical companies have more than five product specifications.
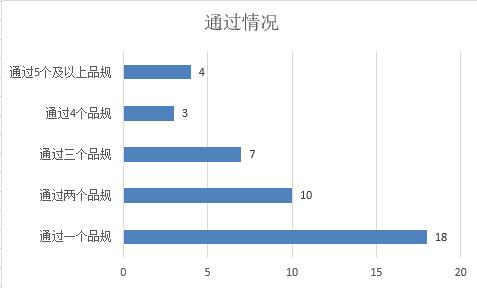
(Source: Cyberblue)
Among them, Zhejiang Huahai Pharmaceutical Co., Ltd. passed the consistency evaluation, while Yangzijiang Pharmaceutical Co., Ltd. and Unacon Co., Ltd. passed the consistency evaluation, and Hengrui Pharma Co., Ltd. passed the consistency evaluation. Zhejiang Jingxin Pharmaceutical, Haosen Pharmaceutical and Zhengda Tianqing all passed the consistency evaluation.
The consistency evaluation of Zhejiang Huahai Pharmaceutical, Yangzijiang Pharmaceutical and Unacon has made rapid progress.
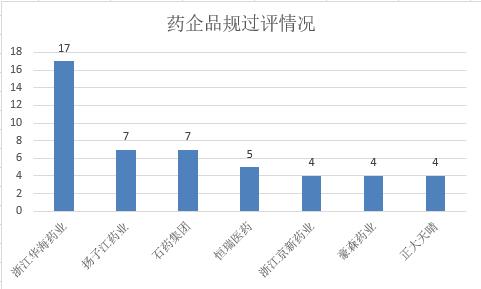
(Source: Cyberblue)
Huahai Pharmaceutical Co., Ltd.: Consistency Evaluation of Overtaking in Curves
According to the annual report of Huahai Pharmaceutical, Huahai Pharmaceutical has invested more in the over-evaluation research and development of irbesartan tablets, fosinopril tablets, lisinopril tablets and losartan potassium tablets.
The annual report shows that in 2017, the company’s R&D expenditure was 438.2377 million yuan, a year-on-year increase of 20.38%, accounting for 8.76% of the operating income of that year. The main reason is that the company has continuously increased the declaration and conformity evaluation of domestic new products, increased the R&D investment in biopharmaceuticals, new drugs, first imitation drugs, innovative pharmaceutical preparations, and accelerated the declaration of new products and models in markets such as the United States and the European Union.
Compared with other enterprises with more varieties, Huahai Pharmaceutical is characterized by adopting the form of "overtaking in corners"
—— Its overseas drugs with quality regulations enter the domestic market through supplementary application for domestic listed varieties and direct application for four kinds of new drugs overseas, and only need to BE declared with overseas listed test data, which will greatly reduce the cost and time of declaration compared with similar products enterprises that need to do BE experiments in China.
For example, its valsartan tablet is the first oral preparation of Huahai Pharmaceutical, which has been listed in Europe and America, and it is deemed to have passed the consistency evaluation if it is declared with overseas data.
Cebolan made statistics on the disclosure announcement of Huahai Pharmaceutical. Except for its product "Voriconazole Tablets", its R&D cost is an important basis for the company’s sales pricing in the US market. The important cost data is corporate trade secrets, and the R&D investment data has not been disclosed.
The total R&D investment of the remaining 9 evaluated varieties of Huahai Pharmaceutical is 44.08 million.
Among them, valsartan tablets have the largest R&D investment of 16.6 million, followed by donepezil hydrochloride tablets with 9.25 million R&D investment.

(Source: Cyberblue)
Unacon: Both are the first enterprises that have been appraised.
According to the statistics of Cybertron, Unacon has passed 7 product specifications and 5 varieties.
Unacon’s semi-annual report shows that the company has been actively promoting consistency evaluation. At the same time, make full use of the opportunity brought by consistency evaluation, strive for greater market share for products, and establish strategic cooperation with core distributors to expand and sink the terminal market to primary medical institutions.
Unacon didn’t disclose its R&D expenses, but according to the statistics of Cybertron, all the evaluated varieties in Unacon were "enterprises that passed the conformity evaluation for the first time".
Metformin hydrochloride: According to the data, metformin, as the most widely used oral hypoglycemic agent in clinic, has been crowned as the "magic medicine". Therefore, according to the statistics of Medical Rubik’s Cube, as a 289 variety, there are as many as 130 drug approval numbers for metformin, including 116 metformin hydrochloride tablets, 8 metformin hydrochloride enteric-coated tablets, 4 metformin hydrochloride enteric-coated capsules and 2 metformin hydrochloride capsules. The consistency evaluation of metformin is bound to be a "drama".
Among them, Unacon submitted its domestic listing application on the condition of "listing in the United States". On July 24th, Unacon’s metformin hydrochloride tablets obtained the registration approval of CFDA, and were officially approved for listing, taking the lead in evaluating the consistency of metformin.
Paclitaxel for injection (albumin-bound): In July, Paclitaxel for injection (albumin-bound) produced in Unacon (trade name Ke Aili) has been included in the Catalogue of Listed Drugs in China. Ke Aili has completed the clinical BE test before applying for new drug registration, and it is deemed to have passed the consistency evaluation, so it can be used instead of the original research product. In this declaration, Unacon surpassed Hengrui, who is known as the "First Brother in R&D", and won the first imitation.
Captopril Tablets: On July 3rd, Captopril Tablets (25mg) produced by Unacon Ouyi Pharmaceutical Company passed the evaluation of the consistency of generic drug quality and efficacy. Unacon Ouyi Pharmaceutical Co., Ltd. became the first enterprise to pass the consistency evaluation of Captopril tablets.
Tramadol Hydrochloride Tablets: On May 21st, Unacon announced that the group’s "Chimet" (tramadol Hydrochloride Tablets (50mg)) has been approved by china food and drug administration to pass the consistency evaluation of generic drug quality and efficacy, becoming the first enterprise in China to pass the consistency evaluation of this variety. It is reported that tramadol Hydrochloride Tablets are one of the key products of the group’s analgesic drugs.
Azithromycin tablets: On April 12th, Unacon announced that the group "Weihong" (azithromycin tablets (0.25g and 0.5g)) had passed the consistency evaluation of generic drugs, becoming the first enterprise in China to pass the consistency evaluation. It is reported that azithromycin tablets are one of the key products of the group’s anti-infective drugs.
Yangzijiang Pharmaceutical: Sustained efforts in mid-2018
Up to now, according to the statistics of Cybertron, seven specifications and five varieties of Yangzijiang Pharmaceutical have passed the consistency evaluation, among which five varieties of amlodipine besylate tablets, montmorillonite powder, glimepiride tablets, enalapril maleate tablets and dexmedetomidine hydrochloride injection have been evaluated.
In the carding process, Ceibaran found that all the remaining varieties passed the consistency evaluation from June to September 2018 except Enalapril Maleate Tablets which were reviewed in September 2017, and Yangzijiang Pharmaceutical continued to exert its strength and continuously reviewed in mid-2018.

(Source: Cyberblue)
From this, it can be seen that the style of Yangzijiang Pharmaceutical Co., Ltd. is to accumulate wealth and make thin hair. At a certain stage, it will exert its strength and continuously evaluate many varieties. When the 289 deadline is coming, Yangzijiang may be accumulating strength and making a big move, which is worth looking forward to.
To sum up, 42 enterprises have varieties that have passed the consistency evaluation, among which Huahai Pharmaceutical, Unacon Pharmaceutical and Yangzijiang Pharmaceutical are the top three, followed by Hengrui Pharma Pharmaceutical, Jingxin Pharmaceutical, Haosen Pharmaceutical and Zhengda Tianqing, and the deadline of 289 is coming soon. All pharmaceutical companies should pay close attention to it.
关于作者